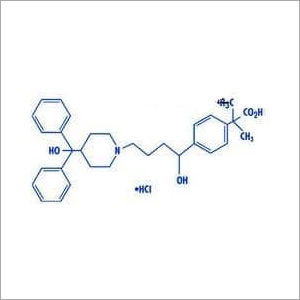
|

Pantoprazole Sodium Sesquihydrate
Product Details:
Product Description
| SL. NO. | TESTS | RESULTS | SPECIFICATIONS |
|
01
02
03
04
05
06
07 |
Description
Solubility
Identification a) By IR b) By HPLC
Water by K.F
Heavy metals
Related Substances (by HPLC% area normalization) a) Single Impurity b) Total Impurity
Assay (% w/w by HPLC, as C16 F2H14 N3 NaO4S on Anhydrous basis) |
Complies
Complies
Complies
5.21% w/w
Less than 20 p pm
0.16% 0.57%
98.43%w/w |
White to almost white colored crystalline powder.
Soluble in Water and practically insoluble in Dichloride methane.
Should match with that of standard. The sample RT should correspond with that if standard
Between 4.5% and 8.0% w/w
Not more than 20 p pm
Not more than 0.5% Not more than 1.0%
Between 98.0% to 102.0% w/w
|
Other Products in 'Active Pharma Ingredients' category
 |
SHEETAL CHEM
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |